Inflammation & Mitochondrial Health
Unraveling the link between inflammation and mitochondrial health: Key insights, cellular dynamics, and therapeutic potential.

June 15, 2023
5 min read
Key takeaways
- Chronic inflammation is prolonged activation of the immune system that is associated with poor health.
- Mitochondria are the site of energy production for cells, and damage to these structures may trigger inflammation.
- Mitophagy is a quality control mechanism that helps to maintain mitochondrial efficiency.
Control of inflammation is a topic of interest when it comes to health promotion. To understand how to best address this, it’s important to understand cell functioning and how the health of the cells can be maintained. This article will explain the role of the mitochondria in inflammation.
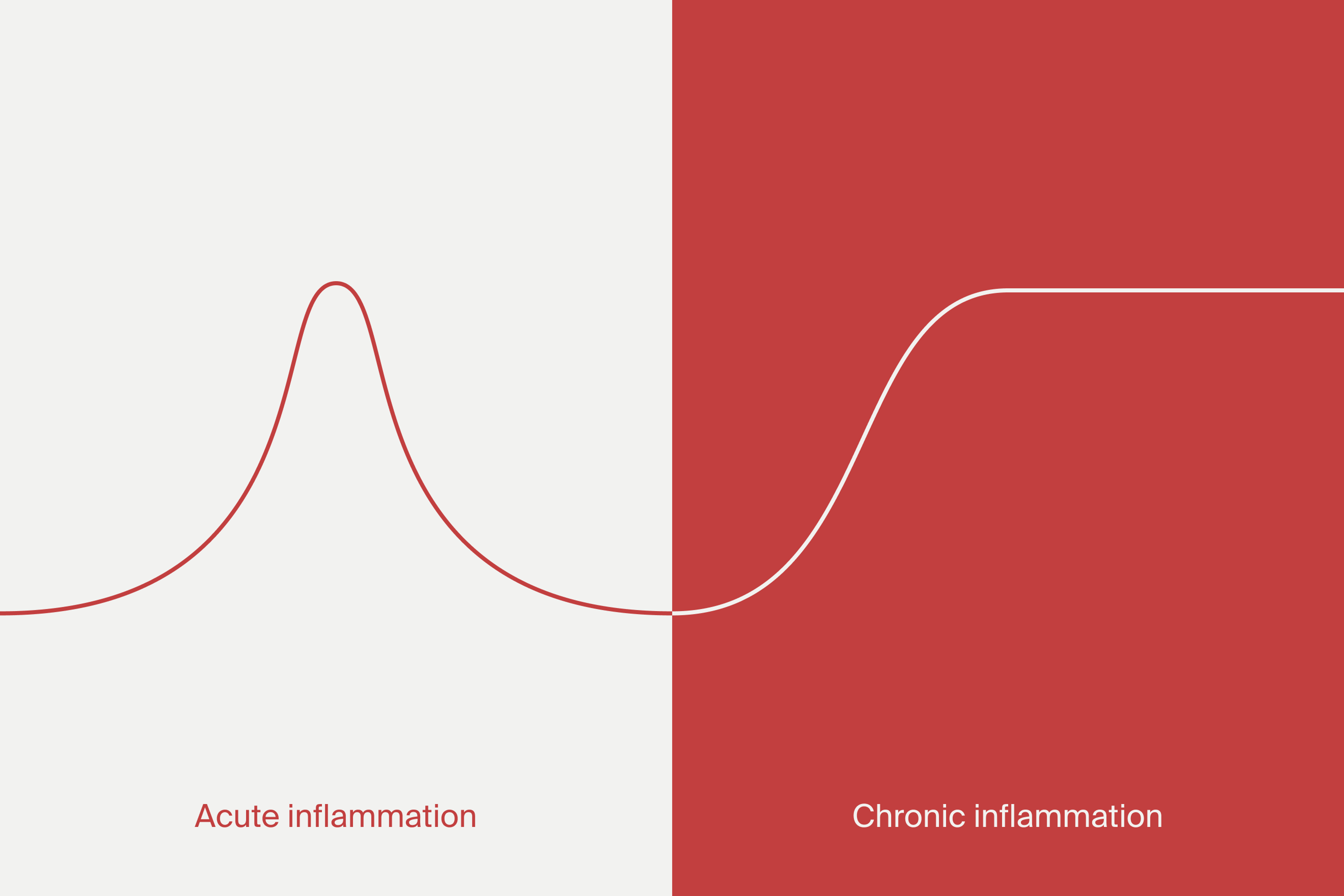
What is chronic inflammation?
Chronic inflammation refers to a prolonged activation of the immune system. Such activation goes beyond the natural levels required for immune function and can result in alterations in cell and tissue function. During aging, low-grade chronic inflammation may develop. This is termed “inflammaging,” which is linked to the development of age-related disease.[1]
Chronic inflammation is associated with several dysfunctions and pathologies, including neuroinflammation, osteoarthritis, and arteriosclerosis.[2] A chronic inflammatory state may also bring tissue degeneration and promote dysfunction of various organs. It has been implicated in the loss of muscle mass in the elderly, dementia, osteoporosis, and cancer and may affect healthy life expectancy.[3]

The mitochondria’s role in inflammation
Mitochondria are considered the “powerhouse” of the cell, as energy needed to power the cell is generated here. Beyond their energy-producing role, mitochondria play a critical role in cellular homeostasis and serve as signaling hubs in various physiological processes, including inflammation.
Mitochondrial dysfunction is one of the biological hallmarks of aging, and mitochondrial defects are often seen in age-related inflammatory conditions. There is an intricate connection between mitochondrial function, innate immunity, and inflammation, and mitochondrial derangement can be considered a crucial pathogenic mechanism for several diseases characterized by chronic inflammation, including neurodegenerative diseases, cancer, rheumatoid and metabolic disorders. When the integrity of mitochondria is compromised, the damage to these structures may trigger inflammation and promote disease.[4]
Numerous processes may occur within the body with mitochondrial dysfunction. For example, when mitochondria become damaged, they release molecules known as damage-associated molecular patterns (DAMPs) into the cell and surrounding environment. DAMPs act as danger signals that alert the immune system to initiate an inflammatory response.[5]
Mitochondria also play a role in oxidative stress as they are a key generator of reactive oxygen species (ROS). While ROS is part of normal cell function, excessive production can lead to uncontrolled reactions with cellular proteins, lipids, and mitochondrial DNA (mtDNA). Such a process disrupts the membrane of the mitochondria and activates an inflammatory response and can cause cellular damage.
Strategies that target optimizing mitochondrial health may play an important role in the pathophysiology of inflammatory conditions.
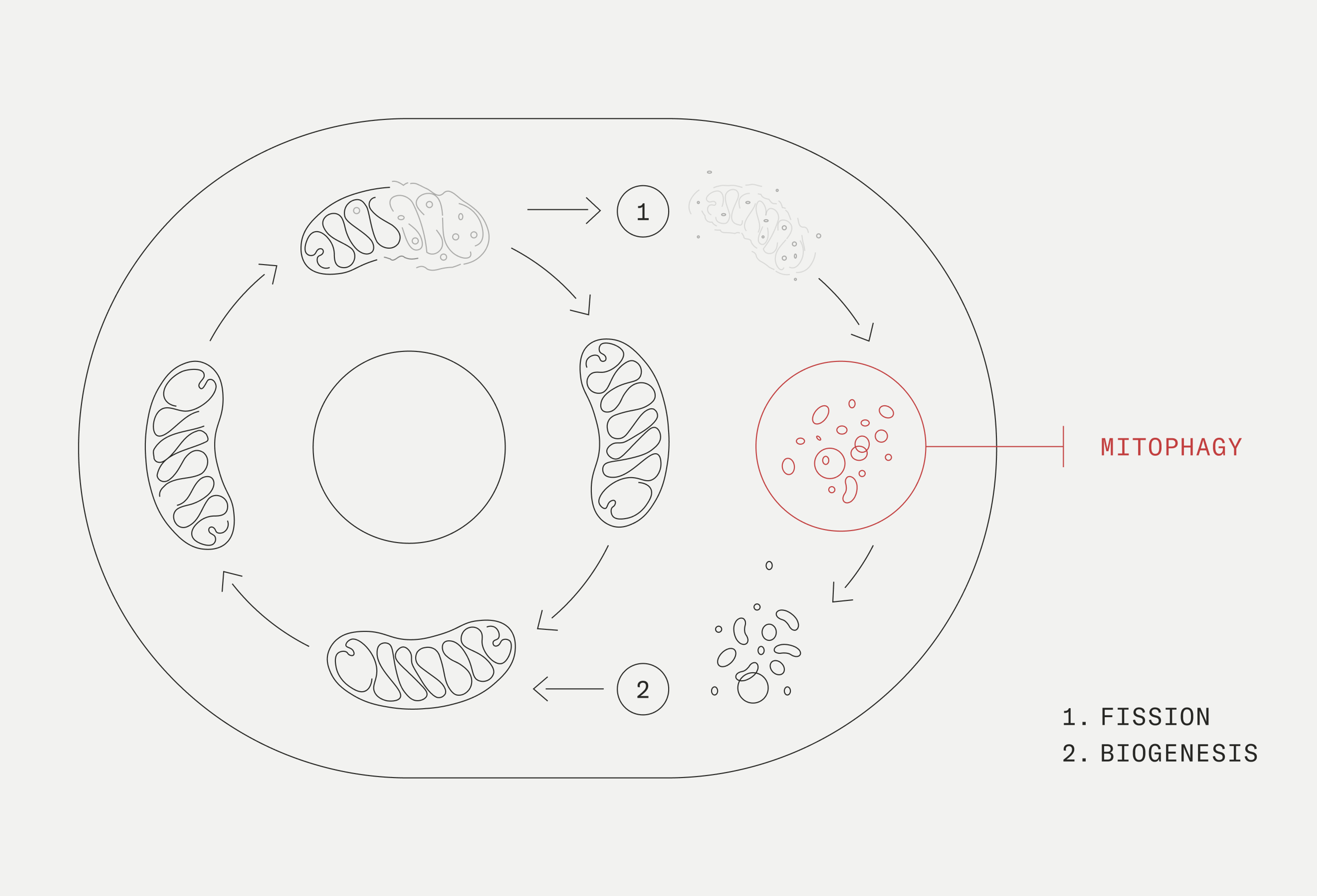
Mitophagy and chronic inflammation
Mitophagy is the process where damaged mitochondria become removed from the cell. Usable components are then recycled into new, better-functioning mitochondria. It is a necessary quality control mechanism to keep our mitochondria functioning efficiently.
When mitochondrial damage occurs, damaged mitochondrial components collect within the cell. Without mitophagy, these damaged components would be released in the cells and bloodstream, triggering an immune response and inflammation. By enhancing mitophagy, we can effectively remove these damage-associated signals and protect against the onset of inflammation.[6]
We still have much to learn about the interplay between mitochondria and inflammation, but new research is expanding our knowledge daily. Finding strategies to reestablish normal mitochondrial physiology and support mitophagy could represent both preventive and life-changing therapeutic interventions for people struggling with chronic age-related inflammatory conditions.
References
- ↑
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018 Oct;14(10):576-590. doi: 10.1038/s41574-018-0059-4. PMID: 30046148.
- ↑
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023 Jan 3.
- ↑
Suzuki K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules. 2019 Jun 7;9(6):223. doi: 10.3390/biom9060223. PMID: 31181700; PMCID: PMC6628010.
- ↑
Sun N, Youle RJ, Finkel T. The Mitochondrial Basis of Aging. Mol Cell. 2016 Mar 3;61(5):654-666. doi: 10.1016/j.molcel.2016.01.028. PMID: 26942670; PMCID: PMC4779179.
Missiroli S, Genovese I, Perrone M, Vezzani B, Vitto VAM, Giorgi C. The Role of Mitochondria in Inflammation: From Cancer to Neurodegenerative Disorders. J Clin Med. 2020 Mar 9;9(3):740. doi: 10.3390/jcm9030740. PMID: 32182899; PMCID: PMC7141240.
West AP. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology. 2017 Nov 1;391:54-63. doi: 10.1016/j.tox.2017.07.016. Epub 2017 Jul 29. PMID: 28765055.
- ↑
Missiroli S, Genovese I, Perrone M, Vezzani B, Vitto VAM, Giorgi C. The Role of Mitochondria in Inflammation: From Cancer to Neurodegenerative Disorders. J Clin Med. 2020 Mar 9;9(3):740. doi: 10.3390/jcm9030740. PMID: 32182899; PMCID: PMC7141240.
- ↑
West AP. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology. 2017 Nov 1;391:54-63. doi: 10.1016/j.tox.2017.07.016. Epub 2017 Jul 29. PMID: 28765055.
Authors
Jinan Banna, PhD, RD
Dietitian, Professor and Writer
Davide D'Amico, PhD
R&D Group Leader at Timeline