Autophagy and mitophagy support cellular health with age
How are autophagy and mitophagy related, and how do they support cellular health? Read on to understand the link and how they promote healthy aging.
December 08, 2023
8 min read
Key takeaways
- Autophagy refers to the breakdown and recycling of old cells in the body and is important for cell functioning
- Mitophagy is a specialized form of autophagy that regulates the breakdown of damaged mitochondria, considered the “powerhouse” of the cell
- Mitochondrial function and autophagy are closely linked
- Both autophagy and mitophagy are important for healthy aging, and defects in these processes may contribute to the development of disease
- Several factors have been shown to be able to induce autophagy and mitophagy
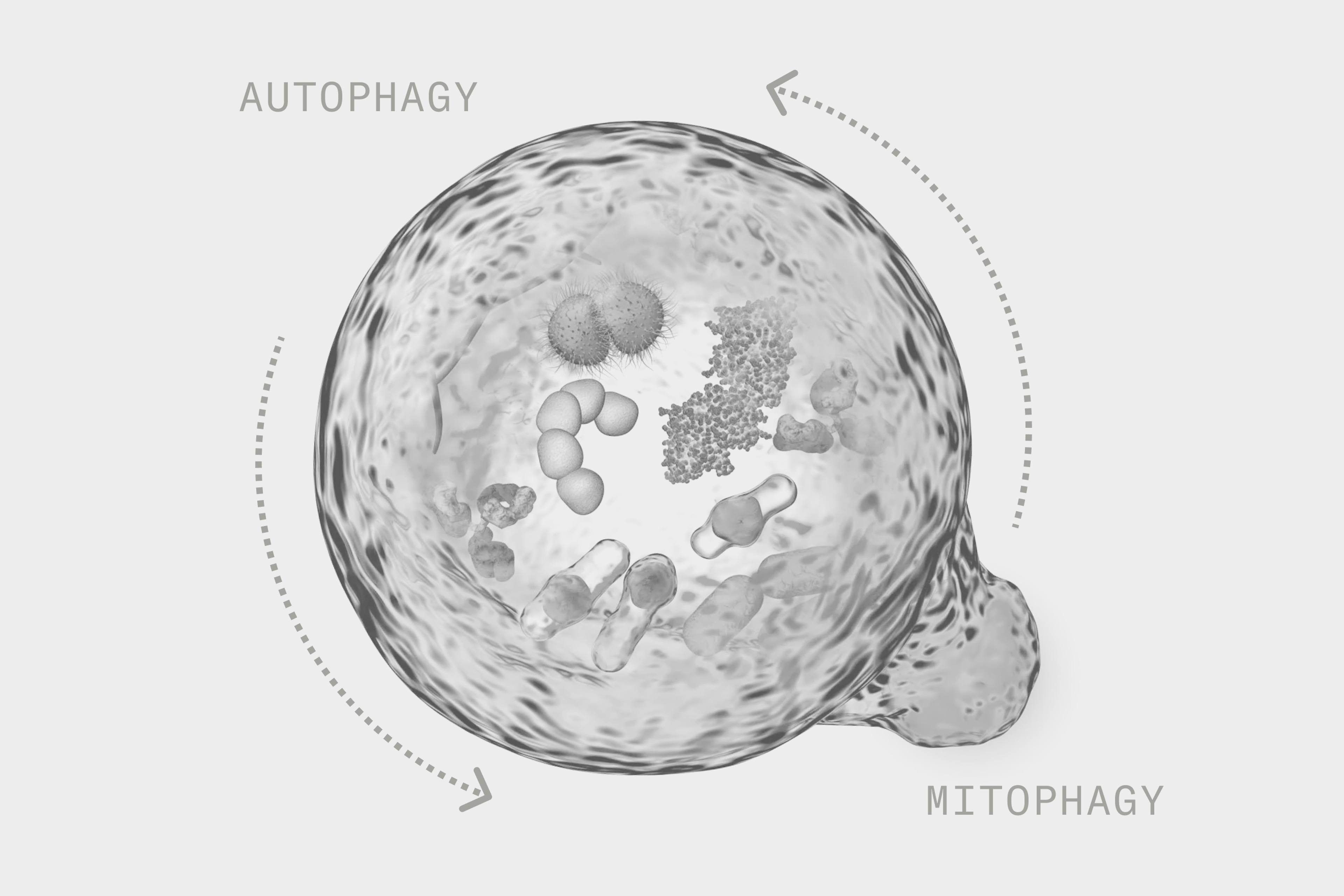
Autophagy and mitophagy support cellular health with age
Promoting healthy aging is a topic of interest for many people, and understanding the processes in the body that relate to health promotion and disease prevention is an important part of this. Autophagy and mitophagy are two such processes that happen at the cellular level and play a critical role in the aging process.
What is autophagy and how does it work in the body?
Autophagy involves the breakdown and recycling of old cells and damaged cell parts and can be considered a rejuvenation system of the cells. Autophagy comes from the Greek language, with 'phagy' meaning eat and 'auto' meaning self. It’s aptly named as autophagy is considered a “self-eating” system necessary for normal cell functioning. Given this important role, it has gained quite a bit of attention when it comes to how it may be used to promote health.[1]
Autophagy is carried out by a class of proteins called autophagy-related proteins. These proteins have been found in many different types of organisms. If this process is disrupted, cellular defects may occur, contributing to a decline in function.[2]
There are three different types of autophagy: macro-autophagy, micro-autophagy, and chaperone-mediated autophagy. Each of these functions distinctly, with the end result being the breakdown and recycling of damaged cell parts.[3]
What is mitophagy and how does it work in the body?
Mitophagy is a specialized form of autophagy that regulates the breakdown of damaged mitochondria. The mitochondria are considered the “powerhouse” of the cell and produce the energy cells need to do their job. Given the important role that mitochondria and mitophagy play in cellular functioning, they have been well-studied with regard to health promotion and disease prevention, as well as longevity.[4]
What is the relationship between activities in the mitochondria and autophagy?
There are several ways in which mitochondrial function and autophagy are linked.
First, problems with mitochondrial functioning have been shown to inhibit autophagy, including mitophagy. This may result in a negative feedback loop that relates to cell aging and death, and the development of disease.[5]
The relationship between mitochondrial activities and autophagy has also been studied under conditions of starvation, which is known to induce autophagy. Mitochondria generate reactive oxygen species (ROS), unstable molecules that may cause cell damage. During a state of starvation, as the ROS build up, they can regulate gene expression involved with autophagy.[6]

Aging and autophagy
How do autophagy and mitophagy relate to aging?
Both autophagy and mitophagy are important areas of focus when it comes to understanding the aging process. Many studies have focused on autophagy dysfunction with age, with a particular emphasis on mitophagy, given its role in energy generation. Turnover of dysfunctional and damaged mitochondria is important for cell health, and impairment of this process can lead to cell death. Defects in autophagy and mitophagy may contribute to physiological markers of aging and chronic disease.[7]
Cell senescence
Aging also leads to cell senescence, which refers to a state in which cells eventually stop multiplying but do not die off. These are often referred to as “zombie cells,” and they release chemical signals that can potentially damage neighboring cells[8].
In senescent cells, autophagy is dysregulated, and this loss of this function may reinforce the senescent state of the cell.[9]
Oxidative stress
Oxidative stress that occurs with age also is closely tied to autophagy, mitophagy, and aging. Cells are continually exposed to ROS, both from the body’s basic processes and from the environment. This can cause damage to the cell, resulting in an impairment in energy production in the mitochondria. [10]Given the age-related dysfunction of autophagy, abnormal mitochondria may accumulate further, increasing oxidative stress.[11]
Oxidative stress is also a cause of DNA damage, which plays a role in the impairment of autophagy. Mitochondrial DNA is particularly vulnerable to ROS. This DNA damage may contribute to cellular senescence and is directly associated with aging and a host of age-related conditions characterized by inflammation.[12]

1. Exercise 2. Diet 3. Polyphenols 4. Urolithin A
How can autophagy and mitophagy be induced?
Given the role autophagy and mitophagy play in healthy aging, there has been a focus on how to induce these processes. Several lifestyle factors have been studied to determine how changes in behavior play a role.
Exercise
Exercise upregulates autophagy in organs and tissues throughout the body, including muscles, the heart, liver, pancreas, and the brain. However, in the muscles, specifically, upregulation of autophagy persists for several days.[13]
Diet
Food quality and quantity, and timing of eating will also influence autophagy and mitophagy.
Calorie restriction, which is generally defined as a reduction in caloric intake below what one normally consumes without depriving the body of essential nutrients, has been heavily researched in terms of autophagy. Several studies show that calorie restriction induces autophagy in several organs and tissues, including the muscle, liver, kidney, heart, pancreatic, and nervous system.[14]
Fasting is another method to induce mitophagy and autophagy. During this practice, you abstain from eating or drinking beverages other than water. A recent review of the literature highlighted research demonstrating that fasting-induced mitophagy in a variety of tissues. [15]
Polyphenols
Polyphenols are natural compounds found in food that have antioxidant and anti-inflammatory properties. Additionally, these compounds have been shown to be autophagy inducers and improve mitochondrial function.[16] Given the many benefits of polyphenols, they are an important target with regard to chronic disease prevention.
Urolithin A
Urolithin A is a nutrient that can stimulate mitophagy[17]. It is produced in the gut after eating antioxidant compounds (ellagitannins and ellagic acid) in foods such as walnuts, pomegranates, and berries. [18]
Urolithin A is not found in the food that we eat. Instead, we must rely on our gut microbiome to make it. Yet the vast majority of us don’t have the right microbiome to make Urolthin A. [19]That’s where supplements may help.
The bottom line
Mitophagy is a specialized form of autophagy targeting the recycling of our mitochondria. Autophagy and mitophagy are key players in human aging, and they will continue to be areas thoroughly studied in longevity research.
References
- ↑
Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011 Nov 11;147(4):728-41. doi: 10.1016/j.cell.2011.10.026. PMID: 22078875.
- ↑
Tran M, Reddy PH. Defective autophagy and mitophagy in aging and Alzheimer’s disease. Frontiers in neuroscience. 2021 Jan 8;14:612757.
- ↑
Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014 Jan 20;20(3):460-73. doi: 10.1089/ars.2013.5371. Epub 2013 Aug 2. PMID: 23725295; PMCID: PMC3894687.
- ↑
Fivenson EM, Lautrup S, Sun N, Scheibye-Knudsen M, Stevnsner T, Nilsen H, Bohr VA, Fang EF. Mitophagy in neurodegeneration and aging. Neurochem Int. 2017 Oct;109:202-209. doi: 10.1016/j.neuint.2017.02.007. Epub 2017 Feb 21. PMID: 28235551; PMCID: PMC5565781.
- ↑
Graef M, Nunnari J. Mitochondria regulate autophagy by conserved signaling pathways. EMBO J. 2011 Jun 1;30(11):2101-14. doi: 10.1038/emboj.2011.104. Epub 2011 Apr 5. PMID: 21468027; PMCID: PMC3117638.
- ↑
Li L, Chen Y, Gibson SB. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signal. 2013 Jan;25(1):50-65. doi: 10.1016/j.cellsig.2012.09.020. Epub 2012 Sep 19. PMID: 23000343.
- ↑
Tran M, Reddy PH. Defective autophagy and mitophagy in aging and Alzheimer’s disease. Frontiers in neuroscience. 2021 Jan 8;14:612757.
- ↑
National Institute on Aging. Does cellular senescence hold secrets for healthier aging? Accessed May 12, 2023. Available at https://www.nia.nih.gov/news/does-cellular-senescence-hold-secrets-healthier-aging (https://www.nia.nih.gov/news/does-cellular-senescence-hold-secrets-healthier-aging)
- ↑
Wiley CD, Campisi J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat Metab. 2021 Oct;3(10):1290-1301. doi: 10.1038/s42255-021-00483-8. Epub 2021 Oct 18. PMID: 34663974; PMCID: PMC8889622.
- ↑
Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular medicine. 2008 Dec;10:291-315.
- ↑
Tran M, Reddy PH. Defective autophagy and mitophagy in aging and Alzheimer’s disease. Frontiers in neuroscience. 2021 Jan 8;14:612757.
- ↑
Tran M, Reddy PH. Defective autophagy and mitophagy in aging and Alzheimer’s disease. Frontiers in neuroscience. 2021 Jan 8;14:612757.
- ↑
Escobar KA, Cole NH, Mermier CM, VanDusseldorp TA. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell. 2019 Feb;18(1):e12876. doi: 10.1111/acel.12876. Epub 2018 Nov 15. PMID: 30430746; PMCID: PMC6351830.
- ↑
Bagherniya M, Butler AE, Barreto GE, Sahebkar A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing research reviews. 2018 Nov 1;47:183-97.
- ↑
Bagherniya M, Butler AE, Barreto GE, Sahebkar A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing research reviews. 2018 Nov 1;47:183-97.
- ↑
Yessenkyzy A, Saliev T, Zhanaliyeva M, Masoud AR, Umbayev B, Sergazy S, Krivykh E, Gulyayev A, Nurgozhin T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients. 2020 May 8;12(5):1344. doi: 10.3390/nu12051344. PMID: 32397145; PMCID: PMC7285205.
- ↑
Jayatunga DPW, Hone E, Khaira H, Lunelli T, Singh H, Guillemin GJ, Fernando B, Garg ML, Verdile G, Martins RN. Therapeutic Potential of Mitophagy-Inducing Microflora Metabolite, Urolithin A for Alzheimer's Disease. Nutrients. 2021 Oct 23;13(11):3744. doi: 10.3390/nu13113744. PMID: 34836000; PMCID: PMC8617978.
- ↑
Andreux PA, Blanco-Bose W, Ryu D, Burdet F, Ibberson M, Aebischer P, Auwerx J, Singh A, Rinsch C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nature Metabolism. 2019 Jun;1(6):595-603.
- ↑
Singh A, D'Amico D, Andreux PA, Dunngalvin G, Kern T, Blanco-Bose W, Auwerx J, Aebischer P, Rinsch C. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur J Clin Nutr. 2022 Feb;76(2):297-308. doi: 10.1038/s41430-021-00950-1. Epub 2021 Jun 11. PMID: 34117375; PMCID: PMC8821002.
Authors
Jinan Banna, PhD, RD
Author
Julie Faitg, PhD